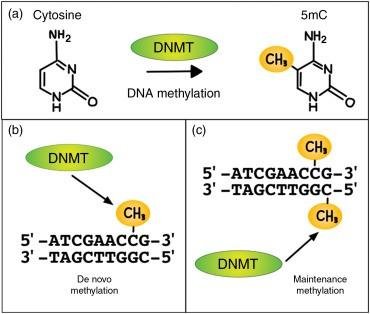
DNA methylation is a pivotal mechanism regulating gene expression, characterized by the covalent attachment of a methyl group to the 5th carbon of cytosine at specific CpG dinucleotide sites mediated by DNA methyltransferases. This process plays a crucial role in DNA replication and transcriptional regulation, encompassing de novo methylation and maintenance methylation pathways. De novo methylation entails the methylation of previously unmethylated DNA strands catalyzed by methyltransferases, whereas maintenance methylation occurs when one strand of the DNA duplex is already methylated, leading to methylation of the unmethylated strand. These methylation modalities collectively maintain the stability and dynamic equilibrium of the methylome.
Principles of DNA Methylation
DNA methylation represents a extensively researched and longstanding epigenetic regulatory mechanism. Broadly, it refers to the chemical modification process where specific bases within the DNA sequence, under the catalytic action of DNA methyltransferases utilizing S-adenosyl methionine as the methyl donor, undergo covalent attachment of methyl groups. This modification can occur at various sites such as the C-5 position of cytosine, N-6 position of adenine, and G-7 position of guanine. In most contexts, It predominantly refers to the methylation process of the 5th carbon of cytosine in CpG dinucleotides. The resultant product, 5-methylcytosine, represents the primary form of DNA methylation in eukaryotes, including plants and animals, and constitutes the sole known form of DNA methylation in mammals. As a relatively stable modification state, DNA methylation can be inherited to offspring DNA during DNA replication via the action of DNA methyltransferases, thus constituting a crucial epigenetic mechanism.
Classification of DNA Methyltransferases
Within the genome, the establishment of DNA methylation patterns relies on the activity of DNA methyltransferases. These enzymes encompass maintenance DNA methyltransferases (also known as Dnmt1 or maintenance methyltransferases) and de novo methyltransferases. Based on sequence homology and function, eukaryotic DNA methyltransferases further subdivide into four classes: Dnmt1/MET1, Dnmt2, CMTs, and Dnmt3. Dnmt1/MET1 enzymes are responsible for maintaining the methylation status of CG sequences. CMTs enzymes are exclusively found in plants, characterized by their catalytic domains T and IV being buried within the chromosomal major area and their specific maintenance of CG sequence methylation. Dnmt3 enzymes have been identified in mice, humans, and zebrafish. Among these, Dnmt3a and Dnmt3b exhibit higher expression levels in undifferentiated embryonic stem cells but lower expression levels in somatic cells. Their primary function is de novo methylation, although they also contribute to maintenance methylation and are responsible for methylation of repetitive sequences.
Detection method of DNA methylation
1) Bisulfite Sequencing
Bisulfite sequencing, a widely employed method for DNA methylation analysis, entails the conversion of unmethylated cytosines to uracils via specific chemical reactions, generating uracil bands in the DNA sequence that are easily discernible. Methylated cytosines remain unaffected, retaining their original state. Subsequently, DNA fragments treated with bisulfite are selectively amplified through PCR. And their sequence information is acquired using DNA sequencing technology. By aligning the sequencing results with the original sequence, one can precisely enumerate the number and distribution of methylated sites, thereby assessing the degree of DNA methylation. This method is particularly suited for detecting methylation levels in individual genes or a limited set thereof.

Principle of the quantitative bisulfite sequencing method (Dimo Dietrich et al,.2019 )
2) RRBS
Reduced Representation Bisulfite Sequencing (RRBS) utilizes bisulfite treatment and restriction enzyme digestion to amplify DNA fragments enriched with high methylation content selectively. This method expedites the accurate detection of heavily methylated CpG sites within the genome.

Study design of Reduced representation bisulfite sequencing (RRBS) (Martin Widschwendter et al,. 2017)
3) WGBS
Whole Genome Bisulfite Sequencing (WGBS) represents a high-throughput approach capable of comprehensively assessing the methylation levels of every individual cytosine base throughout the entire genome. Achieving precise methylation data necessitates deep sequencing coverage with this method.

Whole-Genome Bisulfite Sequencing of DNA Methylomes of Mouse Embryonic Stem Cells (Ehsan Habibi et al,. 2013)
4) MeDIP-seq
Methylated DNA Immunoprecipitation Sequencing (MeDIP-seq) leverages immunoprecipitation with antibodies targeting 5-methylcytosine, succeeded by high-throughput sequencing. This methodology facilitates the comprehensive detection of methylation status across all CpG sites within the genome.

Methylated DNA immunoprecipitation sequencing (MeDIP-seq). (Tibor A. Rauch, Gerd P. Pfeifer, 2019
For more information about DNA Methylation Sequencing Methods, refer to “Overview of DNA Methylation Sequencing Methods”.
Applications of DNA methylation
Disease Pathogenesis
Within the realm of oncology, alterations in the methylation patterns of key tumor-associated genes orchestrate a cascade of events culminating in gene silencing or overexpression, thereby propelling tumorigenesis. Exemplifying this, in breast cancer, frequent DNA methylation events targeting tumor suppressor genes including BRCA1 and TP53 engender diminished or abrogated gene expression, thereby heightening susceptibility to malignancy. Furthermore, deviations in DNA methylation levels intricately correlate with tumor aggressiveness and prognostic outcomes. Investigations underscore that tissues exhibiting heightened methylation profiles typically manifest inferior survival rates and augmented metastatic propensities relative to counterparts evincing lower methylation levels. Consequently, It emerges as a pivotal entity in the landscape of early cancer detection and diagnosis.
Cardiovascular diseases like hypertension, coronary artery disease, and heart attacks are linked to disrupted DNA methylation and gene expression. Besides, DNA methylation aids in diagnosing inherited disorders, including monogenic conditions like cystic fibrosis and hemophilia.
Expanding the purview, emerging evidence implicates DNA methylation in the pathogenesis of psychiatric disorders, including autism spectrum disorder and schizophrenia. Furthermore, investigations suggest a potential nexus between DNA methylation patterns and autoimmune diseases. Such as rheumatoid arthritis and systemic lupus erythematosus, underscoring its multifaceted role in disease pathophysiology.
Exploring the Landscape of Disease Biomarkers
Within the realm of cancer research, DNA methylation emerges as a cornerstone, delineating distinct methylation signatures across diverse malignancies. Interrogating the DNA methylation landscape of neoplastic tissues facilitates early detection, prognostic evaluation, and recurrence surveillance. Noteworthy is the prognostic relevance of gene-specific methylation statuses in lung cancer, colorectal cancer, breast cancer, and other tumor types, elucidating survival probabilities and recurrence propensities.
In the domain of genetic diseases, DNA methylation assumes a diagnostic mantle. For example, By scrutinizing the methylation profiles of select genes, the identification and screening of monogenic inherited disorders, exemplified by cystic fibrosis and hemophilia, are streamlined.
Insights into the causes of psychiatric disorders like autism and schizophrenia are linked to the complex landscape of DNA methylation. Methylation analysis in psychiatric disorders enhances understanding of disease and opens new paths for diagnosis and treatment.
Expanding the purview to autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, burgeoning research underscores the contributory role of DNA methylation. Besides, Evaluation of DNA methylation patterns among patients afflicted with autoimmune disorders augments our comprehension of disease mechanisms and unveils promising therapeutic strategies.
Breeding of Animals and Plants
In the realm of animal and plant breeding, research endeavors delve into the intricate interplay between methylation patterns and various phenotypic manifestations. it includes growth, development, disease resistance, reproductive prowess, embryonic maturation, and senescence. This fundamental exploration serves as a linchpin in understanding the genetic underpinnings of desired traits in both animal and plant species.
Using DNA methylation as a molecular marker gives breeders a powerful tool to identify individuals with desirable traits. For example, disease resistance, pest immunity, and increased productivity in animals and plants. This molecular marker augments precision breeding efforts aimed at enhancing agricultural productivity and sustainability.
Moreover, the advent of genome editing technologies revolutionizes breeding paradigms by facilitating the targeted modulation of methylation profiles within animal and plant genomes. This molecular manipulation engenders precise control over gene expression dynamics, thereby augmenting the manifestation of desired phenotypic traits in progeny.
learn more: dap seq
Environment
Delving into environmental considerations, the utilization of plants for remediation purposes emerges as a promising avenue. Certain botanical species exhibit a remarkable propensity for pollutant sequestration. By modifying the methylation of these plants, their ability to absorb and detoxify pollutants can be enhanced. And it increases their effectiveness in environmental cleanup.
Microbial degradation stands as a cornerstone in the arsenal of environmental remediation strategies. Besides, Manipulating the methylation profiles of microbial consortia holds promise for enhancing their enzymatic prowess in metabolizing organic pollutants, thereby facilitating the bioremediation of contaminated ecosystems.


In the United States, anabolic steroids, together with testosterone and trenbolone, are categorised as Schedule III controlled
substances underneath the Controlled Substances Act.
This legislation, enacted in 1990 and updated in 2004,
makes it unlawful to own, distribute, or use these medicine without
a prescription. Nevertheless, docs can prescribe testosterone legally,
however just for particular medical circumstances such as testosterone deficiency
(hypogonadism) and sure types of anemia. Trenbolone,
then again, is not approved for human use in the united states and is simply allowed in veterinary medicine,
particularly for livestock. Past aggression, some users additionally experience heightened anxiety or depression.
It’s not uncommon for intermediate and even some beginner steroid customers to suppose they’re prepared to leap into a sophisticated cycle simply because there are a few steroid cycles beneath the belt.
This is a doubtlessly grave mistake, though – the final thing you need to
be doing is rushing into superior steroid use when you’re gentle on experience.
Normally, you wish to see the most vital and quickest outcomes
in your newbie steroid cycle. The physique should get well,
which implies ready AT LEAST eight weeks for a new cycle. If you retain doing cycles with little time in between, you could completely mess up your
testosterone operate for good and end up on TRT eternally.
Too excessive of a dosage can lead to muscle loss as a outcome of growing protein metabolism.
You could experience mild side effects at any dose, similar to a higher physique temperature
and elevated hunger (which might not be welcome while cutting).
The timing and frequency of your dosages will
depend upon the esters hooked up to the Trenbolone and testosterone steroids.
Also Trenbolone inhibits the manufacturing of stress hormones
like cortisol which may break down muscle tissue and cause muscle
loss. Trenbolone Acetate and Trenbolone Enanthate are the two
choices mostly used for a tren steroid cycle.
One Other essential a part of any bodybuilder’s arsenal is testosterone post-cycle remedy.
So, you may want to start with a lower dose of
testosterone and enhance it as wanted. When stacking trenbolone with testosterone,
it is necessary to remember that trenbolone is a stronger drug than testosterone.
This is the most typical Trenbolone mixture
since Testosterone is a light drug that will easily be stacked to
considerably boost features without rising Tren’s antagonistic effects.
After discontinuing the use of Tren, the physique sometimes begins to restore its pure testosterone manufacturing, but this can take
some time. This advanced Trenbolone cycle makes a return to the long-estered compounds just for number of clarification to the reader.
Testosterone is the quintessential bodybuilding steroid;
it will increase strength, muscle mass, and general efficiency.
Throughout a Check and Tren cycle, you can count on noticeable will increase in muscle hardness,
density, and definition. The cycle can be identified
for its capacity to boost workout intensity and scale back
body fats percentages. Nevertheless, it’s important to observe for
potential unwanted aspect effects, as both compounds are potent and may
cause opposed reactions if not managed properly. However athletes who stack these two powerful anabolic steroids at greater doses could additionally be
more vulnerable to unwanted side effects.
This is considered one of the explanation why docs issue testosterone alternative therapy (TRT) prescriptions to lots of of hundreds of
men worldwide. Widespread PCT contains medications
like Clomid (clomiphene) or Nolvadex (tamoxifen) for 4–6 weeks,
typically starting 1–2 weeks after the cycle ends, depending
on the esters used. Dosages range based mostly on expertise, however a common cycle would possibly embrace 300–500 mg of
testosterone per week mixed with 200–400 mg
of trenbolone per week. More superior customers may modify dosages, but these amounts are typical for intermediate users.
As Quickly As you’ve had some experience running Test-only cycles,
you possibly can have a glance at further compounds to run. Depending in your aim, a
couple of alternative ways of organising a barely more advanced protocol exist.
Catabolic reactions, similar to breaking down body fats, are the
inverse of anabolic reactions; they metabolize cell parts and
complex substrates to type easier derivatives and launch power.
These are essential to maintain up a steady quantity of substances in the blood.
If you resolve to take less frequent injections, you have to keep in mind the potential of temper swings and a strongly lowered libido.
Beard – As the popularity of rising a beard is again in current times, many people may get pleasure from this “side effect”.
Trenbolone increases the quantity of DHT hormone, which is responsible for stimulating the hair follicles positioned on face.
If you want further hardening effects, add something like Masteron or Anavar
towards the tip of the cycle, as I define in one other stack under.
It may even boost strength into the stratosphere – much more so than Boldenone.
But you additionally get the long listing of Tren unwanted aspect effects that don’t exist with Equipoise.
Some males exclusively use EQ for performance positive aspects and
never necessarily for bulking and even cutting. Unnecessary to say, EQ is a legendary AAS for
growing stamina and endurance. As A End Result Of it’s nearly at all
times stacked with other AAS influencing your
results. These are the core results of Equipoise, and yes, they take some time to kick in, however
guys will at all times be impressed when Boldenone is taking full effect anywhere from the 8th to the 12th-week mark.
This modified model of IGF-1 is engineered to keep away from binding with IGF-binding proteins, extending its half-life to
20–30 hours. As a outcome, it remains active within the physique significantly longer than pure IGF-1.
Every of these alternatives has distinctive mechanisms and benefits, so
deciding on the proper one is decided by your particular goals.
Follistatin-344 – A potent myostatin inhibitor that promotes muscle hypertrophy
by blocking myostatin exercise.
It might help reduce joint pain, an issue we frequently cope with when becoming
more lean throughout slicing. This will depend on several components, but
the primary ones are which steroid compound (or compounds) you need to use and your overall targets (plus if you expect to be faced with any drug testing at any stage).
Steroid cycling is used by people who know precisely what they need to obtain and when, as
well as once they need to be steroid-free when it
comes to being tested. Whether you wish to seem like the Arnie
of yesteryear or wish to get your physique into
peak conditioning with probably the most ripped physique you’ll be able to muster, we know
that many individuals do it with an informed use of steroids.
Steroid customers often stack Trenbolone Enanthate with Primobolan Depot and Testosterone
Propionate. The cycle runs for twelve weeks and can be utilized
by those who have ran cycles female bodybuilders before and After steroids, even when not on the
advanced level.
Zu hohe Testosteronwerte kann man manchmal bei jungen Frauen zwischen dem 20.
Im Rahmen eines Polyzystischen Ovarialsyndroms (PCOS) werden zu viel männliche Hormone in den Eierstöcken der Frauen produziert.
Die Ursache für die hohen Testosteronspiegel bei Frauen mit PCOS
sind noch nicht genau bekannt, genauso wenig wie die Ursache
für die Krankheit. Daneben kann man bei seltenen Fragestellungen noch
das freie Testosteron bestimmen. Normalerweise liegt ein Großteil
des Hormons an Proteine gebunden vor und nur ein sehr kleiner Anteil
des Testosterons findet sich frei im Blut. Freies Testosteron kommt auch im Speichel vor und kann daher anhand einer Speichelprobe gemessen werden. Allgemein fördern hohe Testosteronmengen die Gesichts- und Körperbehaarung, sowohl bei Frauen als auch
bei Männern.
Testosteron, das nicht an Proteine gebunden ist, wird als freies Testosteron bezeichnet und wirkt direkt auf
das Gewebe. Ein zu hoher Testosteronspiegel bedeutet, dass die Menge des Hormons im Blut
die normale Referenzspanne übersteigt. Für erwachsene Männer liegt diese Spanne typischerweise zwischen 300 und 1.000
ng/dl (Nanogramm pro Deziliter), abhängig z.
Wenn der Testosteronwert darüber hinausgeht, spricht man von einer
Hyperandrogenämie, die sowohl natürliche als auch künstliche
Ursachen haben kann (s. u.). Ein erhöhter Testosteronspiegel
kann vorübergehend oder dauerhaft sein und wirkt sich auf
viele Prozesse im Körper aus.
Dazu gehören die Ernährung, körperliche Aktivität, Stress und Umweltfaktoren. Eine ausgewogene Ernährung und regelmäßige
Bewegung können dazu beitragen, einen gesunden Testosteronspiegel aufrechtzuerhalten. Testosteron ist
ein Steroidhormon, das hauptsächlich in den Hoden von Männern und in geringerem
Maße in den Eierstöcken von Frauen produziert wird. Es wird oft als das “männliche”
Sexualhormon bezeichnet, da es für die Entwicklung und
Aufrechterhaltung männlicher Merkmale wie Bartwuchs, Muskelmasse und eine tiefe Stimme verantwortlich
ist.
Gerade wegen Letzterem wird Testosteron immer wieder
als illegales Dopingmittel (Anabolika) benutzt. Testosteron ist aber
nicht nur bei Männern zu finden, sondern auch bei Frauen. Welche Wirkung das Hormon auf unseren Körper hat, wie sich Schwankungen im Testosteronspiegel
auswirken und welche Werte regular sind, erfahren Sie in diesem Artikel.
Das Sexualhormon ist vor allem für Männer und deren Sexleben und die damit verbundene
Libido sehr entscheidend. Ein geringer Testosteronspiegel kann zu erheblichen Problemen führen.
Zeigt das Blutbild beim Testosteron einen dauerhaft erhöhten Wert,
richtet dies einen irreparablen Schaden an der Spermienproduktion in den Hoden an.
Zusätzlich leidet die Psyche am Überschuss, was
zu Konzentrationsstörungen, Depressionen, Stimmungsschwankungen und verminderter Gedächtnisleistung führt.
Diese Angaben in die jeweiligen Einheiten umzurechnen ist aufwendig und nicht
sinnvoll. Die beste Variante ist, das Blutbild mit dem Arzt zu besprechen.
Ein, in einem längeren Zeitraum, erhöhter Testosteronspiegel
hat schwere Nebenwirkungen zur Folge. Sportler, die es mit dem Doping übertreiben, verwenden meist eine zu hohe Dosierung.
Der optimistic Effekt ist, dass der Mann schneller Muskelmasse aufbaut und höhere Leistungen erzielt.
Der Überschuss des Testosterons zieht Schäden mit sich, wie
Herzmuskelschwäche, Leberkrebs, Schlaganfälle und Schilddrüsenüberfunktion. Zu hohe Werte treten auf, wenn die Nebennierenrinden, Hoden oder Eierstöcke, bedingt durch Tumore, übermäßig viel Testosteron ausschütten.
So werden individuelle Wege zu einem ausgewogenen und gesunden Leben ermöglicht.
Das Verständnis des Unterschieds zwischen freiem und Gesamttestosteron ist essentiell, um Deine
Testosteronwerte richtig interpretieren zu können. Diese beiden Parameter bieten tiefe Einblicke
in Deine hormonelle Gesundheit und sind entscheidend für eine gezielte Behandlung bei einem Testosteronungleichgewicht.
Das Hormon wird bei Männern vorzugsweise in den Hoden produziert.
Einfluss auf diese Produktion hat das Luteinisierendes Hormon in den Leydigschen Zwischenzellen, welche sich in den Hoden befinden. Aber auch in der Nebennierenrinde wird das
Sexualhormon gebildet – aber nur in sehr geringen Mengen. Über das Blut gelangt das Hormon dann in die
anderen Organe, im dort seine Wirkung zu entfalten.
frau testosteron estradiol und progesteron zu hoch (Mariano) bestimmt die Ausbildung der männlichen Geschlechtsorgane und steuert die Sexualität und Fruchtbarkeit.
Es ist verantwortlich für die Erektion und die Spermienbildung.
Auch hier ist der Testosteronwert morgens am höchsten und sinkt bis
zum Abend ab. Die Vorteile höherer Testosteronwerte sind zu groß, um sie sich entgehen zu lassen. Möchte man den Testosteronwert bestimmen, sollte die Messung idealerweise zwischen eight und 10
Uhr erfolgen.
Der Großteil des Botenstoffes bindet sich für den Transport an Eiweiße, wie SHBG.
Ist deren Konzentration zu gering, spiegelt sich dies im
Wert des Testosterons wieder. Bei Übergewicht, Erkrankungen der Leber, Niere oder Schilddrüse und bei verschiedenen Medikamenten, führt eine veränderte SHBG-Anzahl zu Messfehlern.
Wer lieber läuft, dem empfehlen wir Steigerungsläufe; also
immer wieder zwischen langsamem Laufen und Sprinten abwechseln. © 2025 Potenz Tipps RedaktionDiese Web Site bietet weder medizinische Beratung an, noch stellt sie Diagnosen oder
Anleitungen für Therapien zur Verfügung. Mit Mitte 20 hatte ich für ganze 2 Jahre mit Potenzproblemen zu kämpfen…
Bis ich nach unzähligen Arztbesuchen und Selbstexperimenten endlich
herausfand, was wirklich hilft.
Malgré cette préférence, les oraux ne doivent pas être
considérés comme dangereux lorsqu’ils sont
utilisés à court docket terme. Lorsque l’huile atteint un vaisseau, des métabolites étrangers pénètrent dans les poumons; ainsi le corps tousse comme mécanisme d’auto-défense pour expulser de telles substances.
En outre, la prise de TUDCA et d’huile de poisson sont
des précautions cruciales pour une safety maximale du cœur et du foie.
En règle générale, la durée d’un cycle doit être égale à la durée du temps de repos des stéroïdes.
Appelez votre médecin pour obtenir des conseils médicaux sur les effets secondaires.
Appelez votre médecin pour obtenir des instructions si vous manquez un rendez-vous pour votre injection de testostérone.
L’injection de testostérone peut affecter les résultats de certains checks médicaux.
La testostérone est une hormone sexuelle naturelle produite dans les testicules de l’homme.
De petites quantités de testostérone sont également produites dans les ovaires et le système surrénalien de la femme.
Il est essentiel de discuter avec un professionnel de la santé pour déterminer la meilleure possibility pour chaque individu.
Leur expérience et leurs connaissances leur permettront d’évaluer le traitement le
plus adapté en fonction des besoins et des préférences de chaque affected person. Il est important de souligner que le dosage optimal du gel de testostérone
peut varier d’un individu à l’autre. Il est donc essentiel
de bénéficier d’un suivi médical régulier pour ajuster la dose en fonction des besoins spécifiques de chaque
personne. Un médecin pourra évaluer les niveaux de testostérone
et recommander le dosage optimum pour obtenir les meilleurs résultats.
Les injections de testostérone peuvent être un traitement efficace pour augmenter les niveaux
de testostérone. Il est essentiel de consulter un médecin pour un suivi
médical régulier et surveiller les éventuels effets secondaires.
Planetesante.ch propose des contenus rédigés dans des termes simples et compréhensibles, régulièrement mis à jour.
Le diagnostic d’un déficit en testostérone (hypogonadisme) est
d’autant plus facile à poser que les symptômes (voir tableau 1)
apparaissent chez un homme jeune. L’hypogonadisme est confirmé quand la
concentration sanguine en testostérone est abaissée lors de
dosages réalisés deux matins consécutifs. C’est également un médicament efficace pour
les athlètes, les haltérophiles et les culturistes, automotive il peut être utile pour développer une masse musculaire
rapide, réduire les graisses corporelles et fournir une plus grande force.
Il améliore également l’endurance et l’apparence physique générale
de l’utilisateur.
Crazy Bulk a enfin fourni une resolution pour la toxicité des
oraux, où les bodybuilders peuvent faire les positive aspects qu’ils désirent en toute sécurité – sans avoir à s’injecter régulièrement.
De , ce n’est généralement pas une bonne idée d’empiler les oraux ensemble,
automobile cela exercera une pression supplémentaire sur
le foie. Bien que la trenbolone soit probablement l’un des stéroïdes
les durs que vous puissiez prendre et qu’elle soit injectable.
Ainsi, ces injections provoquent de forts effets de virilisation chez les femmes.
Selon les conseils d’specialists, les athlètes féminines
devraient éviter les injections de testostérone.
Elle est fabriquée par les testicules chez l’homme et par
les ovaires en moindres quantités chez la femme.
Les effets hépatiques d’Anavar sont Bénin en raison du travail des reins pour traiter
l’oxandrolone, ce qui réduit le stress du foie. De même, l’undécanoate de testostérone ne présente aucun risque, contournant complènt le foie; et au lieu d’être absorbé
par le sys lymphatique.
Ainsi, la plupart des stéroïdes oraux doivent être pris
sans nourriture pour des résultats optimaux. Par exemple, les utilisateurs
qui souhaitent éviter les problèmes de foie peuvent prendre
de la testostérone (injectable) ou de l’andriol (oral) sans problème.
La suppression de la testostérone sera légère (sauf dans le cas de l’undécanoate de
testostérone), et les dommages au foie sont peu probables avec ces trois stéroïdes.
L’Anavar et le Primobolan sont les stéroïdes oraux les plus sûrs lorsqu’on cherche à réduire le taux de graisse corporelle tout en gagnant de la masse musculaire.
De même, l’Undécanoate de testostérone présente peu de risques hépatiques,
automobile il contourne le foie et est absorbé par le système lymphatique.
Dans nos exams, les stéroïdes oraux sont généralement moins bons pour
le cœur, car ils abaissent davantage le taux de
Correlation Cholestérol testostérone (jobdoot.com) HDL
que les injectables.
Le gel de testostérone peut entraîner des réactions inflammatoires sur la peau d’application, tandis que
les injections peuvent entraîner des complications telles que des infections si elles ne sont pas administrées correctement.
Il est necessary de noter que les complications peuvent
varier d’une personne à l’autre et qu’il est essentiel de discuter avec un professionnel de
la santé avant de commencer un traitement. En résumé, le gel de testostérone est une méthode populaire d’administration de la testostérone.
Son utilisation transdermique facilite son application et permet d’obtenir
des niveaux de testostérone stables. Cependant, il est important d’être diligent dans l’administration et de toujours
consulter un professionnel de la santé pour un suivi médical adéquat.
Garlic has been linked to increased testosterone production through a special mechanism than onions.
The energetic compound in garlic, allicin, is known for
its capacity to decrease cortisol ranges, a stress hormone that may
negatively impression testosterone production. Unlike uncooked onions,
which include quercetin and sulfur compounds, garlic primarily works by decreasing
stress-induced testosterone suppression. Some research recommend that combining garlic
and onions in a food plan could create a synergistic effect, potentially enhancing
testosterone levels greater than consuming either alone.
Nonetheless, further clinical trials are needed to establish
their effectiveness in humans conclusively.
In addition, onion peel extracts, wealthy with quercetin, was found to scale back insulin resistance
in male diabetic rats on high fat food plan [62].
Furthermore, streptozotocin-induced diabetic rats administered onion and fenugreek
had decrease oxidative stress levels compared to those administered
only fenugreek [63]. In addition, onion juice was discovered to have an insulin-like action because it decreased blood glucose degree in hyperglycemic animal models [20].
Eggs are rich in vitamin D, cholesterol, and wholesome fat, all of that are essential Best Steroids For Cutting (Empleos.Ssstaffingsolution.Com) testosterone manufacturing.
It also helps hold homocysteine levels down, which can lead to a buildup of this amino acid within the body.
They’re simple to prepare dinner and may be added to many dishes, including meat
and chicken recipes. This makes it much more necessary to keep away
from meals high in nitrates as a lot as attainable.
Nitric oxide additionally helps decrease blood stress
and improves heart well being by making your platelets much less sticky,
which makes them less prone to clump together and type a blood clot that
may cause strokes. In one study, individuals who consumed no much less than 35 kilos of
allium vegetables per yr have been much less prone to develop colon and other kinds of cancers
than those that did not eat them incessantly. This examine was supported by the deanship of research at Jordan University of Science
and Expertise. An aromatase inhibitor (AI) reduces Estrogen ranges
to ease symptoms of excessive estrogen in males.
It is responsible for a wide range of functions, including the event of male sex organs,
muscle development, and the regulation of mood.
Low ranges of testosterone can result in a number of well being issues, similar to reduced libido, low vitality levels,
and despair. The timeframe for seeing an impact on testosterone ranges
can vary from person to person. Some people might discover changes
within a few weeks of frequently consuming onions, whereas others could take longer to expertise any benefits.
Yes, there are several different foods that are known to have potential testosterone-boosting properties,
including garlic, spinach, bananas, and almonds.
Examples of such research approaches is to make the most of particular types of meals or dietary dietary supplements as a
protected and simply reached means. Here, specifically, since 1967 until now, many analysis
studies have revealed the effect of onion on testosterone; nonetheless, this link has yet
to be collectively reviewed or summarized. In addition, a variety of related revealed articles
from the identical databases were included to improve the
integrity of the dialogue, and therefore the edge of the future directions.
Nonetheless, this impact requires additional approval in people, primarily by conducting medical trials.
Apart from potential dietary components, there are a quantity
of life-style adjustments that may assist assist wholesome testosterone ranges.
Aside from the scientific proof, there are additionally quite a few
anecdotal stories from people who claim that consuming onions has helped them increase their testosterone levels.
Nevertheless, these claims are primarily based on private experiences and will not be
relevant to everybody. Cooking could cut back some vitamins but can make others extra bioavailable and the onions simpler to digest.
Onions contain cysteine sulfoxides, which may lower stress levels and enhance sleep,
not directly supporting testosterone health.
If you do cook onions, try to keep the cooking time and temperature
as low as potential to preserve their quercetin content.
Quercetin might help decrease blood stress by boosting how well your blood vessels work and cutting down on inflammation. Lignan consumption through
flaxseed has been proven to considerably cut back LDL and whole cholesterol in studies, she adds.
They are additionally valued for their potential well being
advantages, including their ability to probably affect testosterone levels.
Onions are a common vegetable that is known for its robust
flavor and distinct odor. They are extensively utilized in cooking
and are also recognized for his or her potential health benefits.
One of the areas the place onions have been studied is their
potential to stimulate testosterone manufacturing.
Cooking onions can barely scale back their quercetin content material, however
they will nonetheless retain a big quantity of this beneficial compound.
These include foods rich in zinc, such as pumpkin seeds, oysters, and beef, in addition to foods rich in healthy fats like avocados, olive oil, and nuts.
Common train, stress management, and sufficient sleep are
additionally important factors in maintaining wholesome testosterone ranges.
When it comes to onions, there may be restricted scientific proof to suggest a direct correlation between onion consumption and elevated testosterone ranges.
However, onions do include certain vitamins that may indirectly assist testosterone manufacturing.
Analysis suggests that onions, specifically Allium cepa L., could have a positive impact on testosterone levels in males.
Onions are wealthy in antioxidants and numerous bioactive compounds that may help maintain and enhance testosterone manufacturing.
Mais, il existe aussi des femmes qui subissent ce même cas dès
l’âge de 20 ans. Ce qui implique l’apparition des signes désagréables par rapport à la déformation du
corps automotive le manque de testostérone favorise l’accroissement de l’œstrogène qui accélère le acquire de
poids. Par conséquent, la testostérone hassle également le métabolisme chez
la femme. Sa pressure et sa densité minérale osseuse diminuent et il est
difficile de former de la masse musculaire. La présence d’une bonne focus
de taux de testostérone dans le corps permet d’optimiser le gain de masse musculaire.
Un apport régulier en magnésium peut avoir un impact direct sur la production de testostérone.
En effet, les séances longues et épuisantes avec des temps de repos relativement longs font monter
le taux de cortisol, ce qui a comme effet de
réduire la testostérone. Des recherches scientifiques ont démontré que des périodes
de repos plus courtes entre les séries favorisent la production de cette dernière.
Les recherches ont largement démontré que toutes
les personnes devraient s’assurer que leur
niveau de testostérone est bon.
L’alimentation, l’exercice, la gestion du stress, le
sommeil adéquat, et le maintien d’un poids santé sont tous des
facteurs qui interagissent pour booster naturellement vos niveaux d’hormones.
Commencez avec de petits changements dans votre quotidien; ces
ajustements peuvent non seulement augmenter votre testostérone mais également améliorer votre santé générale et votre
bien-être. L’activité physique, et plus particulièrement la musculation, peut
avoir un impression considérable sur vos niveaux de testostérone.
Les exercices composés qui impliquent de grands
groupes musculaires, comme les squats, les deadlifts, et les presses, stimulent
la production de l’hormone.
Les recherches ont démontré que les glucides nous
permettent d’optimiser le niveau de testostérone pendant les séances d’entraînement.
Les lipides ne sont pas forcément des ennemis,
contrairement à la croyance populaire. Bien que beaucoup
de personnes voient les lipides d’un mauvais œil, tu dois savoir qu’ils jouent un rôle crucial dans la production de
la testostérone. Augmenter son taux de testostérone est une expression qui sent souvent mauvais les produits
illicites et dangereux pour la santé.
Sinon, on risque de manquer d’énergie pendant l’entraînement avec des réserves de glycogène insuffisantes dans les muscular tissues.
C’est vrai que les hommes en produisent 10 fois plus que les femmes en moyenne.
Mais cette hormone stéroïdienne est essentielle à la santé et à
la qualité de vie, quel que soit son sexe.
Le stress est l’un des principaux facteurs de la baisse de
testostérone, automobile il provoque la production de cortisol.
En réduisant les niveaux de cortisol, l’ashwagandha permet à votre
corps de mieux produire de la testostérone. Ce complément est particulièrement recommandé pour les personnes qui sont
souvent soumises à des situations stressantes. Le pyrèthre d’Afrique,
aussi appelé Tribulus Terrestris, est une plante utilisée
depuis des siècles pour améliorer la santé sexuelle et la vitalité.
Il est particulièrement populaire pour son rôle dans l’augmentation de la testostérone.
En effet, cette plante a montré des résultats positifs en termes d’amélioration de
la libido et de stimulation de la production de testostérone.
De plus, elle favorise la santé du cœur et des muscles, ce qui en fait
un choix polyvalent.
Elle influe sur la libido, l’énergie, la masse musculaire, la distribution des graisses, et même l’humeur.
Alors, remark peut-on augmenter naturellement ses niveaux
de testostérone pour améliorer sa qualité de vie? C’est la question que se posent de
nombreux hommes qui cherchent à optimiser leur potentiel, que ce soit signification d’un test sanguin de phosphatase alcaline élevée; https://femdel.com/, level de vue physique, psychological ou
sexuel.
Pour augmenter son taux de testostérone, des coachs sportifs et influenceurs prônent pour la plupart des compléments alimentaires (vitamine D, zinc,
magnésium) ou des cocktails surprenants. Ces ingrédients et minéraux sont facilement
trouvables en ligne sur les websites d’influenceurs sportifs célèbres
ou en pharmacie. Chez l’homme, la testostérone est naturellement sécrétée par les testicules.
On cite souvent le fenugrec, une herbe couramment utilisée en cuisine et en médecine traditionnelle, pour ses effets potentiels
sur la testostérone. Des études ont montré que le fenugrec peut avoir
un impression positif sur la libido et les niveaux de
testostérone, Cela est probablement dû à ses composés appelés saponines stéroïdiennes.
Ces composés pourraient jouer un rôle dans la stimulation de la
manufacturing de testostérone. Bien que les bonnes graisses
soient importantes pour la santé hormonale, il est
essentiel de les consommer avec équilibre et modération. Une alimentation trop riche en graisses,
même saines, peut entraîner un déséquilibre nutritionnel et
des problèmes de santé. Il est donc necessary d’adopter
une approche équilibrée en intégrant ces graisses dans le cadre
d’une alimentation variée et équilibrée. Aussi, le zinc, abondant dans
les huîtres et les graines de citrouille, s’avère indispensable sur ce
terrain.
Au contraire, cela augmente le taux de cortisol ou hydro-cortisone qui
est la cause du blocage de la sécrétion de l’hormone mâle.
Le temps idéal pour une bonne manufacturing est les entrainements à courte durée, voir
1 minute. Cependant, l’intensité des exercices de musculation permettent vraiment la stimulation de la testostérone.
Les répétions forcées, les drop-sets, les répétitions partielles sont les méthodes à pratiquer pour booster la testostérone.
Les séances de HIIT cardio OU Haute Intensité Intervalle Coaching sont également très conseillées.
Deca is a slow-acting steroid that helps to construct muscle mass and improve power.
Both Deca-Durabolin and testosterone can be utilized to build
muscle, however they work in numerous ways. Testosterone promotes
muscle progress by stimulating the body to store more nitrogen,
whereas Deca will increase the variety of pink blood cells, which leads to elevated endurance and strength.
These are water retention and bloating which cannot be avoided, however
work fantastic in a bulking and power gaining cycle.
This will simply preserve the body’s normal Testosterone functioning during the time when the
body’s natural manufacturing of Testosterone is being suppressed.
Some customers go longer but this adds to the risk of adverse unwanted
effects and fewer gains, plus an elevated danger of damaging the Hypothalamic Pituitary Axis (HPTA).
This characteristic is most well-liked by many users of Nandrolone, particularly those utilizing Deca-Durabolin cycles since it is a slower and longer performing compound.
Deca-Durabolin is an intramuscular injection, and the
best place to administer it is amongst the massive muscle
tissue.
Dianabol may be stacked with trenbolone; nonetheless, it typically causes bloating and
thus counteracts trenbolone’s diuretic properties (similar to Anadrol).
Thus, our sufferers make the most of Nolvadex and Clomid after
this stack to resurrect testosterone ranges (without the addition of hCG).
Winstrol does not have a high androgenic rating; nevertheless,
it does trigger notable androgenic results in our experience.
Due To This Fact, pimples, oily pores and skin, and hair loss are to be expected when combining it with trenbolone.
We have seen this duo utilized as a slicing cycle, where customers eat
in a calorie deficit.
However, it is necessary to notice that each steroids have their very own distinctive benefits and downsides,
and the only option will depend upon the user’s targets
and particular person physique chemistry. Deca Durabolin, also called nandrolone decanoate, was created
by Organon in 1960 and hit the market in 1962.
It rapidly turned a top choice in bodybuilding, thanks to its capacity to increase muscle measurement
and hasten recovery. Deca improves nitrogen holding
and protein building within the physique, main to raised performance for bodybuilders.
Lastly, no discussion about steroid cycles can be complete
without addressing the potential side effects.
To view it, please log in to verify your age.By
persevering with, you also agree that use of this website constitutes
acceptance of Reddit’s Person Agreement and
acknowledgement of our Privacy Coverage. While
a Check E and Deca cycle can produce wonderful outcomes, it’s important to concentrate on potential unwanted effects and manage them effectively.
Testosterone Enanthate is amongst the most commonly used
forms of testosterone for both therapeutic and performance-enhancing functions.
Bodybuilders have to weigh the good in opposition to the dangerous, considering carefully
about their health and objectives. Important recommendation includes frequently checking your health
to ensure safety. The purpose is to make use of steroids safely to get the most effective outcomes with out
risking your well being.
The thigh and buttocks are probably the most beneficial injection sites, and you need to rotate your injection muscle tissue to permit recovery.
Avoid injecting Deca-Durabolin into small muscles and areas with arteries.
The injection website should be swapped with an alcohol
wipe to clean earlier than injecting to take care of
hygiene and forestall an infection. PCT on deca must be similar to
PCT on a testosterone cycle; nevertheless, while using Deca, it’s a good
suggestion to make use of AI corresponding to Arimidex or Aromasin. Utilizing low to average doses should not be an issue, even with no aromatase
inhibitors, however it’s higher to be secure than sorry.
Such myths become a actuality with a trenbolone/Winstrol cycle, the place a user’s waist measurement decreases and muscle size increases simultaneously.
Unfortunately, the unwanted effects rival the
advantages of this cycle, being extraordinarily harsh.
Thus, this cycle is only for very skilled steroid customers who
are snug dealing with heavy compounds. Trenbolone is actually an injectable steroid utilized by bodybuilders to realize large quantities of lean muscle and energy whereas enhancing fat loss
(1). Don’t anticipate to hit the fitness center as viciously when you are off your steroid
cycle Pros and cons of Testosterone pills (https://eduxhire.com/) operating publish cycle therapy.
Individuals might really feel drained or not so pumped as a lot
as workout but that’s only pure. Preserving a optimistic mindset when running submit
cycle remedy is essential.
The benefit of gaining any power is that you could be pushing your most lifts larger for faster positive aspects.
With Deca-Durabolin’s ability to strengthen joints and pace up restoration, you need to be able to cut down that point
between working every muscle group by a day or
two. Many guys who speak about impressive strength gains with Deca-Durabolin are stacking it with no less than testosterone, and infrequently over 600mg per week of testosterone.
Testosterone will significantly boost your strength, in order that stacked mixture will take your
energy to another degree, much more so than utilizing Deca-Durabolin alone.
If you’re going for a 16 week cycle, take the testosterone supplement, HGH and Arimidex during the cycle.
If you tried this take a look at and deca cycle and didn’t really feel any disagreeable unwanted effects, you might do the cycle again but
this time add 30 mg of dianabol every day and zero.5
mg of Arimidex each different day. One of the unwanted aspect
effects of deca is it slows down your body’s testosterone
production.
Ester is principally a «tail» added to the chemical structure of the substance.
It doesn’t change the consequences however alters the half-life,
aka the time your physique needs to break it down and actually USE the substance.
It reveals how just 200mg of Testosterone Enanthate (or Cypionate) can provide
you an extra 67% of red blood cell rely. In fundamental terms, it’s literally the identical
compound that your balls produce, but it comes from an injection (aka exogenously) to offer
you all the advantages of an enhanced Testosterone stage. Because something
that basic can be an excellent alternative to elucidate all of the stuff intimately.
After all, Check solely cycle is a well-liked first steroid cycle option for newbies.
Would love to hear doses you’ve all experimented with as well has how your results had been.
Using any data provided by the website is solely on the viewers’ discretion. The Drug Enforcement
Administration (DEA) classifies AAS as Schedule III medicine.
Just possessing them illegally (not prescribed to you by a doctor) can result in up to a 12
months in jail and a fantastic of no much less than $1,000
for a first-time offense. Nonetheless, it doesn’t outcome in the muscle-building claims this drug’s advertising copy might
lead you to imagine. Look out for any extra ingredients in dietary supplements that may have unwanted effects or
trigger allergic reactions.
It has the potential to vary lives, and improve the health
of an individual in so many ways. Bodybuilders often use anabolic steroids to realize their goals to be a champion of their
sport, and it can be a fantastic alternative for them.
When you buy anabolic steroids, you automatically create a positive nitrogen balance in your body, which speeds up protein synthesis in your cells.
As a end result, there is a noticeable improve in muscle mass, which continues long after the cycle
is over. There are no much less than 32 forms of totally
different anabolics that you can be discover on a industrial website.
One thing that you want to know about anabolics is that they’re also
used for medical functions as well.
In muscle cells, anabolic steroids enter the nucleus and change how a lot of certain proteins are made.
Proteins that are concerned in building muscle are upregulated, that means
the steroids ‘up’ the number of them being made.
Proteins that are concerned in breaking down muscle are downregulated, which
means much less of them are made. Discover a variety of SARMs designed to enhance muscle progress, pace up restoration,
and enhance total athletic efficiency. Every SARM we offer is lab-tested and quality-assured on your safety.
The liquid steroids for sale in our catalog can be found in the
form of either disposable ampoules or reusable vials all containing high-quality components.
Injectable steroids are undoubtedly the most popular type
of steroid use within the USA and elsewhere all over
the world.
We are delighted to hear to that the products you obtained lived as a lot
as your expectations, and that our team was in a place to offer you attentive and responsive customer
service. The updates are supplied on the net site when they are obtainable.
There could possibly be a while lags, that is understood as products take time to move.
It depends on the product’s ingredients, however generally,
every formula promotes healthy testosterone production and prevents testosterone
from transforming into estrogen over time in males.
We are not in the business of convincing you to make use of the steroids we’ve on the
market, however solely present you with the choices obtainable to you.
Each month, pharmaceutical corporations surprise the world with new pharmacological therapies,
as we’re all aware. Adding these new formulation to our inventory isn’t something
we’re dashing to do till we’ve confirmed that they’ve passed
formal checks and have been reviewed for high quality and effectiveness first.
In this case, you do not even want to go away your house or the fitness center.
There’s never been a better place to purchase steroids online
in Dubai. Our online store presents athletes dependable anabolic and androgenic
steroids that outperform all competitors. High Quality anabolic steroids
for sale are simple to find in our store. The Roids USA staff are well-informed about
legitimate anabolic steroid manufacturers and only
cope with respected corporations. Every pharmaceutical product that enters the Roids USA system
is totally examined and evaluated, and we collect suggestions
on the drug before offering it to shoppers. You could also be assured that your transactions with
us will go smoothly since we by no means provide anabolic steroids for sale that we are not sure of.
Roids USA shoppers, who’re largely athletes of all ranges and kinds, have previously proved the efficacy
of testosterone-boosting medicine.
We supply retail from one of the best pharmacies out
there corresponding to; Meditech, LA pharma, Alpha pharma, Axiom
Pharma and Platinum Biotech. As my first time buying
here, it actually stunned me how good they are.
From communication to payment to delivery to products high quality and effectiveness, everything was
top notch. We are accustomed to setting lofty targets
since many people are maximalists.
Authorized steroids have emerged as a popular alternative, offering a natural various to achieve desired outcomes without compromising
well being. Zentec Performance presents top of
the range mail-order Anabolic steroids for sale in Canada for building
energy and fats loss. Order at present to obtain the highest high quality merchandise to assist your progress.
Prime bodybuilders and athletes in Canada use our merchandise,
serving to them attain their desired gains and
weight loss required for competitors. The right use of anabolic steroids is along side a nutritious diet and common exercise routine to extend muscle mass and energy.
These Legal steroids For weight loss (genome-tech.ucsd.edu)
alternate options are popular amongst many bodybuilders and athletes and supply maximum results
when paired with a wholesome life-style.
The energetic substance can remain in the physique for ten days or more.
Unfortunately, because of the relatively excessive price ketoglutarate can not be replaced with a glutamine complement novorapid.
If you wish to have perfect and powerful physique, be sensible and buy anabolics on-line.
70918248
References:
post steroid cycle supplements (https://www.quimka.net/a9360-%D7%97%D7%9C%D7%90%D7%AA-%D7%94%D7%9E%D7%99%D7%9F-%D7%94%D7%90%D7%A0%D7%95%D7%A9%D7%99/)
70918248
References:
weight gain steroids Tablets – sabarinews.com
–
70918248
References:
Jon skywalker steroids
70918248
References:
none
70918248
References:
none
70918248
References:
alcohol and prednisone, shkolnaiapora.ru,
thief river falls casino
References:
https://Old.newcroplive.Com/Video/planet-startups-oct-3-2020/
is a steroid a hormone
References:
how is synthetic Testosterone Made, posteezy.com,
dianabol dosage cycle
References:
Dianabol Anavar Cycle (Cerealhead9.Bravejournal.Net)
dianabol test cycle
References:
dianabol cycle before and after – https://enregistre-le.Top/item/404511,
recommended dosage of hgh for bodybuilding
References:
hgh legal kaufen
dangerous drugs that build muscle
References:
Dianabol cost (intensedebate.com)
how often do you inject cjc 1295 ipamorelin
References:
can you mix cjc 1295 and ipamorelin
warrior stack – bpc157+tb500+cjc1295+ipamorelin
References:
ipamorelin bacteriostatic water (logisticconsultant.net)
does cjc ipamorelin work
References:
Tesamorelin and cjc-1295 mod grf 1-29 and ipamorelin 12mg
dosage [https://findnearbyjob.com]
cjc 1295 ipamorelin kidney
References:
valley.md
ipamorelin 2mg dosage
References:
side effects of ipamorelin and cjc (https://sewajob.com)
what’s better ipamorelin or sermorelin
References:
tesa + ipamorelin + cjc 1295 blend
can i take cjc 1295/ipamorelin in the morning
References:
cjc 1295 ipamorelin vs hgh
dosing for ipamorelin
References:
Ipamorelin 2mg vial, charmed-serial.online,